GLP-1 analogues for obesity?
NO
PHARMACEUTICAL INFLUENCE
GLP-1 analogues for obesity?
Obesity, we can all agree, is a vitally important issue underpinning many causes of mobility and mortality. The risks have come into sharp focus during the present pandemic. But do you ever feel frustrated with the limited options for treatment?
Consider John, a 45yr old man with a BMI of 36. His father suffered an MI in his early 50s. John has come for advice as he is understandably worried about his own risks now that he is reaching a similar age. He has a BP of 163/92 and a HBA1C of 44, putting him in the pre-diabetic range. You tackle the tricky topic of his weight. John reports he has tried ‘all the diets’ over the years with no lasting improvement, ‘nothing works’. You summon your best motivational interviewing skills and encourage John to attend a local tier 2 service for structured support with diet and exercise. He agrees to give it a go - hurrah!
Six months later he returns demoralised. His weight has barely changed. However, John has seen the news about a new ‘wonder drug’ for obesity. ‘What do you think about it Doctor? Can I have it?’
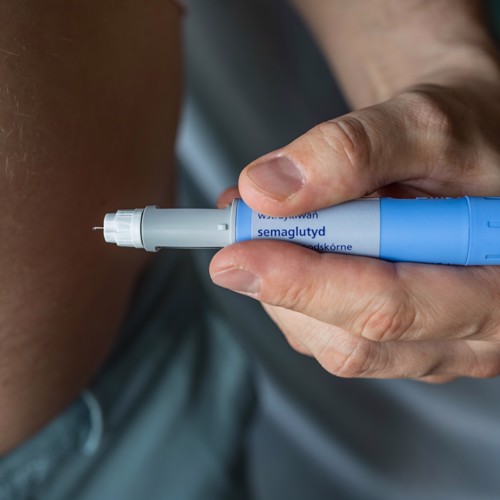
The drug in question is Semaglutide, a once-weekly injectable GLP-1 analogue currently used for diabetes management. The evidence triggering this headline is a recently published double blinded RCT in which weekly high-dose semaglutide (2.4mg) plus lifestyle intervention was compared to placebo plus lifestyle intervention. The semaglutide group lost an impressive average of 14.9% body weight over a 68 week period vs 2.4% in the placebo group. It is worth noting that a significant proportion of those in the semaglutide group (74.2%) experienced gastrointestinal side effects, with nausea being the most common. Although the study reports that the majority of these were mild/moderate (and only 4.5% discontinued treatment due to GI side effects) you do wonder if this had an impact on the weight loss...
So can John have it? The short answer is no: semaglutide has not been approved for obesity treatment yet. However NICE has recently approved liraglutide, a once-daily GLP-1 analogue injection, for managing obesity alongside a calorie-controlled diet and increased physical activity (TA664). The trial data for liraglutide shows a more modest but significant effect on weight loss (-4.32% weight relative to placebo over a 3 year period of use (95% CI -4.94 to- 3.7). Importantly for John, they also report a delay in the development of type 2 diabetes and reduction in CVS disease.
NICE have set the following criteria for use of liraglutide in obesity:
- BMI >35 (or >32.5 if from a minority ethnic group at higher risk of complications of obesity)
AND
- Presence of non diabetic hyperglycaemia (i.e. prediabetes)
AND
- At high risk of cardiovascular disease based on risk factors (e.g. hypertension and dyslipidaemia)
Prescription must be via a tier 3 weight management service (to ensure appropriate diet, lifestyle, and psychological support…plus the NHS gets a discount when prescribed through this route!)
What does this mean for John? There are key limitations to the evidence. Firstly a treatment duration of 2 years is proposed. There is no data about what happens to weight after stopping treatment. Given that obesity is a chronic condition there are clear questions to be asked about the long-term benefits on weight management. Furthermore, cardiovascular benefits were inferred from surrogate measures such as a reduction in HBA1C and BP rather than primary outcomes, which makes any clinical benefit more uncertain.
So he is eligible for a new treatment for now, liraglutide, via tier 3 weight management services. But if the duration of treatment is limited will he come back to us in the same situation in 5 years time?
Dr Laura Darby
17th March 2021
Join us at one of our upcoming courses or live webinars

Recent NB Blogs
Need an immediate update? – all our courses are available on demand
Did you find this useful?
You can quickly add CPD to your account by writing a reflective note about the GLP-1 analogues for obesity? post you've read.
Log in to your NB Dashboard and use the 'Add Reflective Note' button at the bottom of a blog entry to add your note.

